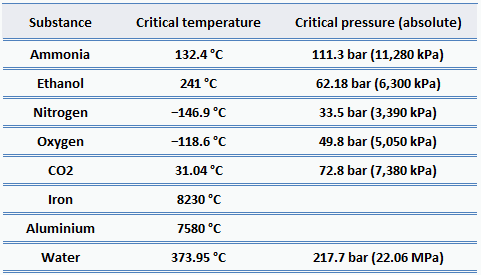
What Is The Critical Pressure Of Water. Ammonia NH 3 4055. - 3048794 dexterassault15 dexterassault15 17092020 Science Senior High School answered What is the critical pressure of water. Propane C 3 H 8 370. Critical Temperature K Critical Pressure MPa Critical Pressure atm Hydrogen H 332.

On the other hand physical properties such as density. At pressure that is higher than the critical pressure of water water is in special state that is known as supercritical fluid state. Note that at or above 374oC the critical temperature for water only water vapor exists in the tube. The critical temperature for a pure substance is the temperature above which the gas cannot become liquid regardless of the applied pressure. At pressures above the critical pressure properties of water in the reactor change gradually and continuously from those we ordinarily associate with a liquid high density small compressibility to those of a gas low density large compressibility without a phase change. At higher temperatures the gas cannot be liquefied by pressure alone.
At the critical point defined by the critical temperature T.
At the critical temperature the. Superheated steam can be used above the saturation limit. At higher temperatures the gas cannot be liquefied by pressure alone. The pressure required to liquify a substance vapor at its critical temperature. Critical pressure and temperature of water are 220 bar and 37314 C as steam water is a valuable heating agent below 200 C where the saturation pressure is about 24 bar. What is the critical pressure of water.