Like Dissolves Like Chemistry. Like dissolves like means if any substance is polar then polar and ionic substances will be dissolved in it. Salt NaCl is ionic which is considered extremely polar. Distilled water food coloring apron corn starch vegetable oil Canola oil beakers beaker tongs glucose hot. Watch more of this topic httpcltchus1FcyyF3GET MORE CLUTCHVISIT our website for mo.

In chemistry the like dissolves like rule refers to solubility and polar and non-polar substances. Like dissolves like is an expression used by chemists to remember how some solvents work. Iodine and carbon tetrachloride are both nonpolar so the liquid dissolves the solid. This paper describes a demonstration and a straightforward experiment using the guided-inquiry approach for the like dissolves like principle that is implemented at the beginning of the academic year. An error occurred while retrieving. Water will not dissolve oil.
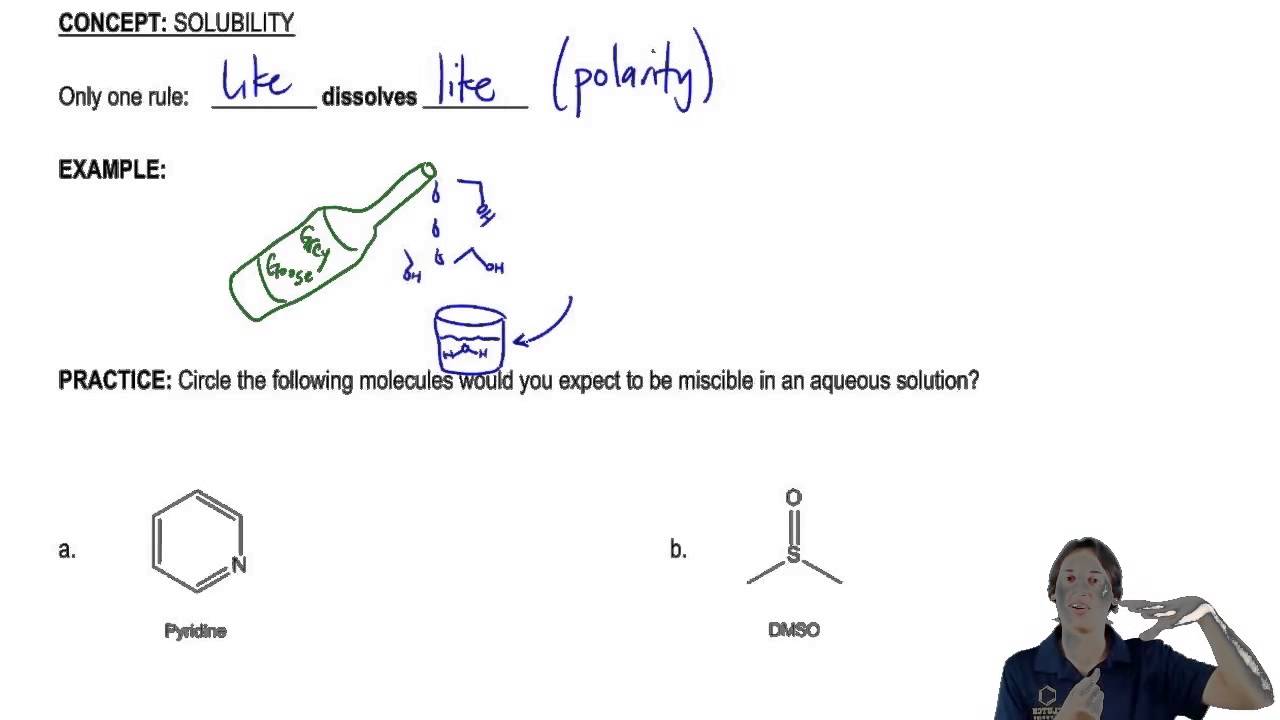
This experiment extends the students understanding of the concept of solubility of organic molecules and its relationship to chemical structure.
Polar solutes dissolve in polar solvents. Miscibility and dielectric constant are fundamental properties that govern the applications of liquid mixtures. Solutions Physical Chemistry Organic Bonding Background and Uses This is about as easy a demo to do as there is and it has good Real World Connections. Water will not dissolve oil. Only one rule to know here. Specifically polar solvents tend to dissolve polar solutes and non-polar solvents tend to dissolve non-polar solutes while non-polar and polar substances are Immiscible do not mix.