
Grignard Reagent Synthesis Mechanism. 000185 mol 26034 gmol 04816 g Percent yield 09806 g 04816 g X 100 2036 Discussion. It is a non-chain radical reaction. Triphenylmethanol was prepared using a Grignard reagent in this experiment. The solution turns cloudy begins to boil and the magnesium metal gradually disappears.
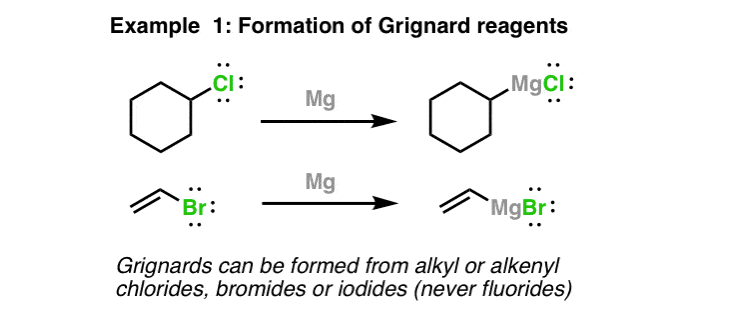
Grignard Reagent Reaction Mechanism and ShortcutNeed help with Orgo. Phenylmagnesium Bromide was used as the Grignard reagent. MECHANISM OF GRIGNARD REACTION The first step in the Grignard reaction is the nucleophilic addition of Grignard reagent to the polar multiple bond to give an adduct which upon hydrolytic workup gives the final product like alcohol. Once the Grignard reagent phenyl magnesium bromide is formed the benzophenone is added. 1 day agosynthesis of 2-methyl-2-octanol using Mg HBr acetone When forming a grignard reagent a reflux is obtained without directly adding heat. As already stated Grignard reagents form via the reaction of an alkyl or aryl halide with magnesium metal.
The solution turns cloudy begins to boil and the magnesium metal gradually disappears.
Q Note that the first step is rate-determining and involves the transfer of one electron from Mg which has two electrons in its valence shell to the carbon-halogen bond. Phenylmagnesium Bromide was used as the Grignard reagent. Dodocane is a possible side product that can form while preparing this grignard reagent. This synthesis begins with the formation of the Grignard reagent from bromobenzene in ether in the presence of crushed magnesium turnings. MECHANISM OF GRIGNARD REACTION The first step in the Grignard reaction is the nucleophilic addition of Grignard reagent to the polar multiple bond to give an adduct which upon hydrolytic workup gives the final product like alcohol. Grignard formation does not involve a radical chain mechanism.